Merkezi Sinir Sistemi Tümörleri/Paraganglioma
Görünüm
| Bu sayfa başka bir dilden çevrilmektedir.
Çekingen davranmayın:
|
Overview
[değiştir]- Paragangliomas are still sometimes called glomus tumors (not to be confused with glomus tumors of the skin) and chemodectomas, but paraganglioma is the currently accepted and preferred term
- A rare neoplasm that can be found in the abdomen (85%), thorax (12%), and in the head and neck region (3%)
- Incidence estimated at 1:1,000,000
- Categorized as a neuroendocrine tumor
- Usually considered benign and complete surgical removal results in cure. However, in about 3-5% of cases they are malignant and have the ability to metastasize.
- Most occur as single tumors. When they occur in multiple sites they are usually found as a part of a heritable syndrome such as MEN types II-A and II-B and Carney syndrome.
Inheritance
[değiştir]- Familial paragangliomas account for ~25% of cases, are often multiple and bilateral, and occur at an earlier age.
- Mutations of the genes SDHD (previously known as PGL1), PGL2, and SDHC (previously PGL3) have been identified as causing familial head and neck paragangliomas.
- Mutations of SDHB play an important role in familial adrenal pheochromocytoma and extra-adrenal paraganglioma (of abdomen and thorax), although there is considerable overlap in the types of tumors associated with SDHB and SDHD gene mutations.
Pathology
[değiştir]- Arise from the glomus cells, which are special chemoreceptors located along blood vessels that have a role in regulating blood pressure and blood flow.
- The glomus cells are a part of the paraganglion system, composed of the extra-adrenal paraganglia of the autonomic nervous system, derived from the embryonic neural crest. Thus, paragangliomas are a type of neuroendocrine tumor, and are closely related to pheochromocytomas. Although all paragangliomas contain neurosecretory granules, only about 1-3% have clinical evidence of oversecretion.
- According to the WHO classification of neuroendocrine tumors, paragangliomas are classified as having a neural cell line of origin. In the categorization proposed by Wick, the paragangliomas belong to Group II.
- The main concentration of glomus cells are found are in the carotid body and the aortic bodies
- Individual tumor cells are polygonal to oval and are arranged in distinctive cell balls, called Zellballen. These cell balls are separated by fibrovascular stroma and surrounded by sustenacular cells.
- The paragangliomas appear grossly as sharply circumscribed polypoid masses and they have a firm to rubbery consistency. They are highly vascular tumors and may have a deep red color.
- With IHC, the chief cells located in the cell balls are positive for chromogranin, synaptophysin, NSE, serotonin and neurofilamen; they are S-100 negative. The sustenacular cells are S-100 positive and focally positive for GFAP. By histochemistry, the paraganglioma cells are argyrophilic, PAS negative, mucicarmine negative, and argentaffin negative.
Clinical characteristics
[değiştir]Paragangiomas are described by their site of origin and are often given special names:
- Carotid paraganglioma (carotid body tumor): Is the most common of the head and neck paragangliomas. It usually presents as a painless neck mass, but larger tumors may cause cranial nerve palsies, usually of the vagus nerve and hypoglossal nerve.
- Glomus tympanicum and Glomus jugulare: Both commonly present as a middle ear mass resulting in tinnitus (in 80%) and hearing loss (in 60%). The cranial nerves of the jugular foramen may be compressed, resulting swallowing difficulty.
- Vagal paragangliomas: These are the least common of the head and neck paragangliomas. They usually present as a painless neck mass, but may result in dysphagia and hoarseness.
- Other sites: Rare sites of involvement are the larynx, nasal cavity, paranasal sinuses, thyroid gland, and the thoracic inlet.
Treatment
[değiştir]- The main treatment modalities are surgery, embolization and radiotherapy.
- When radiation is given, doses are in the range of 45-50 Gy.
- For stereotactic radiosurgery, median margin dose (50% isodose) used is ~16 Gy
Glomus Jugulare
[değiştir]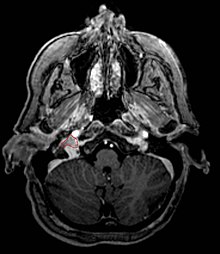
Overview
[değiştir]- Typically considered slow-growing (estimated doubling time 4.2 years) benign tumors, <5% malignant potential
- However, they are locally agressive neoplastic lesions, involving bony erosion and destruction of neurovascular structures
- Commonly arise from the paraganglia of the jugular bulb
- Typically invade the tympanic cavity and jugular foramen
- Can extensively invade petroclival region
- Can invade cavernous sinus above
- Can invade hypoglossal canal below
- Clinical presentation typically with tinnitus or hearing loss, but may also impact jugular foramen CNs
- Glasscock-Jackson Classification:
| G-J Class | Description |
|---|---|
| I | Small tumor involing jugular bulb, middle ear, and mastoid |
| II | Tumor extending under internal auditory canal; may have intracranial canal extension |
| III | Tumor extending into petrous apex; may have intracranial canal extension |
| C1 | Tumor extending beyond petrous apex; into clivus or infratemporal fossa |
- Fisch Classification:
| Fisch Class | Description |
|---|---|
| A | Tumor limited to the middle ear cleft |
| B | Tumor limited to the tympanomastoid area, with no infralabyrinthine involvement |
| C | Tumor invading infralabyrinthine compartment and petrous apex |
| C1 | Tumor with limited involvement of the vertical carotid canal |
| C2 | Tumor invading the vertical carotid canal |
| C3 | Tumor invading the horizontal carotid canal |
| D1 | Tumor with intracranial extension <2cm |
| D2 | Tumor with intracranial extension >2cm |
- Optimal treatment strategy is not clear, with multiple options:
- Surgery: primary option if brainstem compression, or in young patients with functional CN loss. Surgical morbidity not insignificant
- Fractionated RT: typical doses 45-55 Gy, mechanism of action likely related to fibrosis of feeding vessels and not direct glomus cell destruction. Complication rate low
- SRS: No long-term experience yet, but appears a good option due to high conformality. RT dose typically ~16 Gy at 50% margin isodose. Typically ~1/3 have volume shrinkage, and ~2/3 no change, <10% further growth. Clinically ~50% may experience improvement
- Embolization: typically considered an adjunctive treatment to surgery or RT. Not adequate alone due to poor tumor coverage and frequent re-vascularization
Stereotactic Radiosurgery
[değiştir]- Verona, 2006 (Italy)(1996-2005) PMID 16955038 -- "Glomus jugulare tumors: the option of gamma knife radiosurgery." (Gerosa M, Neurosurgery. 2006 Sep;59(3):561-9; discussion 561-9.)
- Retrospective. 20 patients (3 primary GKS, 8 recurrence post surgery, 11 recurrence after embolization). Average volume 7 cm3, mean marginal dose 17.3 Gy (13-24). Estimated doubling 4.2 years. Mean F/U 4.2 years
- Outcome: Improved volume 40%, no change 55%
- Toxicity: Improved CN function 25%, no neuro change 65%, hearing loss 10%
- Conclusion: GKS effective, negligible side effects
- Virginia, 2005 PMID 15662818 -- "Gamma knife surgery for glomus jugulare tumors: an intermediate report on efficacy and safety." (Sheehan J, J Neurosurg. 2005 Jan;102 Suppl:241-6.)
- Retrospective. 8 patients. Median margin dose 15 Gy (12-18). Median F/U 2.7 years
- Outcome: clinically 100% stability or improvement; radiographically 4/7 decreased, 3/7 stable
- Toxicity: None
- Conclusion: Effective local control and preservation of neurologic function
- Mayo Clinic, 2004 PMID 15329025 -- "Stereotactic radiosurgery in patients with glomus jugulare tumors." (Pollock BE, Neurosurg Focus. 2004 Aug 15;17(2):E10.)
- Retrospective. 42 patients treated with GKS. Mean volume 13.2 cm3. Mean margin dose 14.9 Gy. Mean F/U 3.7 years
- Outcome: 31% decreased, 67% unchanged, 2% grew. PFS at 7 years 100%, at 10 years 75%
- Toxicity: 15% new deficit (hearing loss, facial numbness, vocal cord paralysis, vertigo). Hearing preservation 81% at 4 years
- Conclusion: GKS good tumor control, safe
- Stanford, 2004 PMID 15329026 -- "Efficacy and safety of stereotactic radiosurgery for glomus jugulare tumors." (Lim M, Neurosurg Focus. 2004 Aug 15;17(2):E11.)
- Retrospective. 13 patients with 16 tumors. Treated with LINAC/Cyberknife to 14-27 Gy. Median F/U 3.4 years
- Outcome: clinically 100% stable; radiographically 100% decreased or stable
- Toxicity: one transient ipsilateral vocal cord paralysis (however, patient also had prior EBRT)
- Conclusion: RS effective and safe
- Vienna, 2001 (Austria)(1993-1999) PMID 11696882 -- "Efficiency of gamma knife radiosurgery in the treatment of glomus jugulare tumors." (Saringer W, Minim Invasive Neurosurg. 2001 Sep;44(3):141-6.)
- Retrospective. 12 patients. All Fisch Class D. Mean F/U 4.2 years
- Outcome: clincally 50% improved, 50% no change; radiographically decreased 25%, stable 75%
- Toxicity: transient cranial neuropathy in 17%.
- Conclusion: GKS attractive treatment option
- European Multicenter, 1999 (1992-1998) PMID 10592113 -- "Gamma Knife radiosurgery of the glomus jugulare tumour - early multicentre experience." (Liscak R, Acta Neurochir (Wien). 1999;141(11):1141-6.)
- Retrospective. 6 sites, 52/66 patients. Median margin dose 16.5 Gy (10-30). Median F/U 2 years
- Outcome: clinically 29% improved, 65% stable; radiographically decreased 40%, stable 60%
- Conclusion: safe, with no acute morbidity; need longer follow-up
- Graz, 1999 (Austria)(1992-1998) PMID 10536716 -- "Gamma knife radiosurgery for glomus jugulare tumours." (Eustacchio S, Acta Neurochir (Wien). 1999;141(8):811-8.)
- Retrospective. 10/13 patients with imaging followup. Median marginal dose 13.5 Gy to 50% isodose line. Median F/U 3.1 years
- Outcome: 40% decreased volume, 60% unchanged. Improved clinical status in 50%, stable in 50%
- Toxicity: none
- Conclusion: effective treatment option
- Prague, 1998 (Czech Republic)(1993-1997) PMID 9782246 -- "Leksell gamma knife radiosurgery of the tumor glomus jugulare and tympanicum." (Liscak R, Stereotact Funct Neurosurg. 1998 Oct;70 Suppl 1:152-60.)
- Retrospective. 14 patients. Mean maximum dose 37.4 Gy (20-44); mean margin dose 19.4 Gy (10-25). Mean F/U 1.7 years
- Outcome: radiographically 30% improved, 70% stable; clinically 36% improved
- Toxicity: worsening hearing in 21%
- Conclusion: GKS safe treatment; will need long follow-up
Surgery vs. SRS
[değiştir]- Meta-analysis, 2004 (1994-2004) PMID 15329019 -- "Comparison of radiosurgery and conventional surgery for the treatment of glomus jugulare tumors." (Gottfried ON, Neurosurg Focus. 2004 Aug 15;17(2):E4.)
- 7 surgical series 374 patients, 8 GKS series 142 patients. Mean F/U surgery 4.1 years, GKS 3.3 years
- Outcome: local control surgery 92%, recurrence 3%; GKS decrease 36%, stable 61%, recurrence 2%
- Toxicity: surgery CSF leak 8%, mortality 1.3%; GKS morbidity 8%, no mortality
- Conclusion: Both treatments safe and efficacious; surgery higher morbidity but long term GKS outcomes unknown
Kaynakça
[değiştir]- Pellitteri PK et al. Paragangliomas of the head and neck. Oral Oncology 2004 Jul;40(6):563-75. (PMID 15063383)
- Sukhamay Lahiri, "Aortic body", in AccessScience@McGraw-Hill
- John T. Hansen, "Carotid body", in AccessScience@McGraw-Hill
- Wick MR. Neuroendocrine neoplasia. Current concepts. Am J Clin Pathol. 2000 Mar;113(3):331-5. (PMID 10705811)
- The Otology Group
