Biyokimya/Metabolizma ve enerji
Metabolizma
[değiştir]Anabolizma ve katabolizma
[değiştir]Metabolizma (Şekil 1) yaygın tabirle besinin enerjiye, hücre yapılarına ve atık ürünlere dönüşümüdür.

Şekil 1: Metabolizmaya genel bakış
Yukarıdaki diyagram metabolizmanın farklı parçalarını göstermektedir:
- enerji kaynağı, her şeyden önce, enerjisi fotosentez yoluyla kullanılan güneştir.
- katabolizma, besinin kimyasal enerjiye yıkımıdır.
- anabolizma, küçük çevresel moleküllerden kimyasal enerjiyi kullanarak karmaşık hücresel moleküllerin üretimidir.
Katabolik tepkimeler enerji açığa çıkartır, bu yüzden egzergonik (enerji veren) olarak tanımlanırlar; fakat anabolik tepkimeler enerjiyi tüketirler ve bu yüzden endergonik (enerji alan) olarak tanımlanırlar.
Yüksek enerjili fosfatlar
[değiştir]Besin bileşenlerinin çok çeşitli olması ve anabolizmada enerjiye ihtiyaç duyan biyokimyasal tepkime sayısının fazla olması sebebiyle belirli bir anabolik tepkimeyi, katabolizmadaki belirli bir enerji kaynağıyla eşleştirmek oldukça yetersiz olacaktır. Onun yerine hücre, bir ara bileşik, bir çeşit evrensel enerji birimi kullanır. Bu ara madde yüksek enerjili fosfat olarak adlandırılır.
Ancak bir fosfat rubu ne zaman "yüksek enerjili" olur, "düşük enerjili" fosfatla aralarındaki fark nedir? Açığa çıkan şey hidrolizin ΔG0'sidir. Hidroliz bir bileşikten su ekleyerek fosfatı ayırır:
O O R-OP-OH + H2O ⇌ R-OH + HO-P-OH O O
Düşük enerjili (inorganik) fosfatın ΔG0''ı (Pi olarak adlandırılır) 9-20 kJ mol-1'dir, fakat yüksek enerjili fosfatın (Ⓟ ile gösterilir) ΔG0''ı ~30 kJ mol-1'dir.
pKa değeri
[değiştir]Ⓟ'ı bu kadar özel yapan şey nedir? Bunu açıklamak için, pH ve pKa değerlerininin ne olduğuna bakmalıyız. Bir fosfat grubu sıfırla üç arasında OH grubuna sahiptir. Bu da Ⓟ'a, içinde bulunduğu çözeltinin pH grubuna bağlı olarak dört farklı formda (0, 1, 2 ve 3 OH grubu içeren, Şekil 2) bulunma olanağı verir. pKa değeri ise moleküllerin yarısı bir formda (mesela bir OH grubu içeren) diğer yarısı da başka bir formda (mesela 2 OH grubu içeren) ikenki pH değeridir. Bu Henderson-Hasselbalch eşitliği ile ifade edilir:

Şekil 2: Bir fosfat grubunun dört formu pKa2 hücre içindeki koşulları temsil eder.
Peki Ⓟ ve PPi' nin birbirlerinden farkı nedir? Bir ROⓅ'nin ester bağının kırılması, bir PPi bağının kopmasından daha fazla enerji açığa çıkarır (Şekil 3). Bunun sebepleri vardır:
- PPi deki iki fosfat grubu arasındaki elektrostatik itme
- PPi ile karşılaştırıldığında iki Pi grubunun rezonans stabilizasyonu (Şekil 4)

Şekil 3: Ⓟ ve PPi'nin hidrolizi

Şekil 4: Pi'nin rezonans stabilizasyonu
Rezonans stabilizasyonu, hem OH hem de =O'nun fosfat etrafında "hareket edebileceği" anlamına gelir. Elbette bu kaba bir benzetmedir; gerçekte hareket etmezler, elektronlar sadece fosfat atomunun etrafına "bulaşır". Bu aynı zamanda ⇌ yerine ↔ okunun kullanılmasıyla da gösterilir; üç form aslında gerçekte mevcut değildir, sadece kimyasal gerçekliği yazmanın bir yoludur.
Şekil 3'te görebileceğiniz gibi, PPi⇌2Pi için ΔG0 değeri ≪0'dır ve reaksiyonu 2Pi lehine kaydırır.
Yüksek enerjili fosfatlar kullanan moleküller
[değiştir]Fosforik asit ve karboksil grubu arasındaki anhidrit
[değiştir]Hidroliz : ΔG0' = -49.3 kJ mol-1

Guanidin fosfat
[değiştir]Hidroliz : ΔG0' = -43.0 kJ mol-1

Enol fosfat
[değiştir]Örneğin, fosfoenolpiruvat
Hidroliz : ΔG0' = -61.9 kJ mol-1
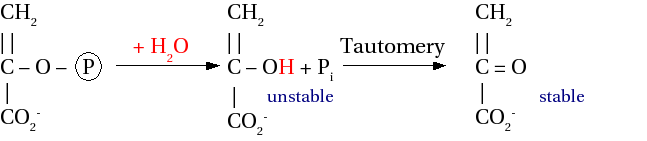
ATP
[değiştir]Adenozin trifosfat, bir düşük enerjili ve iki yüksek enerjili fosfat bağı içerir:

Düşük enerji : ΔG0' = -14,2 kJ mol-1
Yüksek enerji : ΔG0' = -30.5 kJ mol-1
- ATP, ADP (adenozin difosfat), Pi ve enerjiden (gıdalardan) yeniden üretilir; İşlem sırasında H2O salınır.
- ATP, hücrenin kısa vadeli enerji "para birimi" dir.
- Hücredeki ATP konsantrasyonu düşüktür (ATP: 2-8mM; ADP: 0,2-0,8mM). ATP döngüsü yüksek seviyelerde sürdülür.
- ATP, kimyasal işini eşli reaksiyonlar yoluyla gerçekleştirir.
- Eşli reaksiyonlar her zaman Ⓟ transferleridir, asla doğrudan hidroliz değildir.
Temel olarak, ATP kaynaklı herhangi bir reaksiyon tersine çevrilebilir ve süreçte ADP ve Pi'den ATP oluşturur. Ancak, bazı ATP kaynaklı reaksiyonlar asla tersine çevrilmemelidir; bunlar nükleotid ve protein sentezini içerir. Bunlar tersine çevrilirse, organizma enerji için kendi DNA'sını ve proteinlerini parçalara ayıracaktır, bu oldukça talihsiz bir strateji. Asla tersine çevrilmemesi gereken reaksiyonlar için ATP, AMP (adenozin monofosfat) ve PPi'ye -ve PPi'de 2×Pi'ye- bölünebilir. Bu reaksiyon, in vivo koşullar altında tamamen geri döndürülemez olan -65,7 kJ mol-1'lik bir 'G0' değerine sahiptir.
AMP'nin tekrar doğrudan ATP'ye dönüştürülemeyeceği unutulmamalıdır. Bunun yerine, AMP kinaz enzimi, bir ATP ve bir AMP'den iki ADP molekülü oluşturur. Ortaya çıkan ADP'ler daha sonra yukarıda açıklandığı gibi işlenir.
Kovalent olmayan bağlar
[değiştir]Kovalent bağların yok edilmesi büyük miktarda enerji gerektirir. Bir O 2 molekülünün iki oksijen atomuna parçalanması için ~ 460 kJ mol -1 gerekir. Bu nedenle, "canlı" biyokimyanın hiçbir yerinde kovalent bağlar gerçekten yok edilemez; biri kırılırsa bir başkası oluşturulur. Bununla birlikte, birçok biyokimyasal fonksiyon, zayıf/ ikincil/kovalent olmayan bağlar olarak adlandırılan bağları kullanmaktadır.
Zayıf bağlar, kovalent bağlardan çok daha kolay oluşturulur ve yok edilir. Zayıf bir bağı yok etmek için gereken tipik enerji aralığı 4-30 kJ mol-1'dir. Bu nedenle, zayıf bağların oluşumu enerji açısından elverişlidir, ancak bu bağlar aynı zamanda kinetik (termal) enerji (moleküllerin normal hareketi) tarafından da kolayca kırılır. Biyokimyasal etkileşimler genellikle geçicidir (örneğin, bir substrat, işlendikten hemen sonra bir enzimi terk etmelidir) ve zayıf bağların zayıflığı bu tarz etkileşimlerde çok önemlidir. Ayrıca, biyokimyasal özgüllük (örneğin enzimin substratı tanıması), zayıf bağların temel özelliklerinden ikisi kullanılarak elde edilir:
- Bireysel zayıf bağlar zayıf olduklarından, birçoğunun aynı zamanda kabaca aynı yerde belirli bir modelde meydana gelmesi gerekir.
- Zayıf bağlar kısa menzillidir.
Üç temel zayıf bağ türü ve dördüncü bir "sözde bağ" vardır:
İyonik bağlar
[değiştir]İyonik bağlar, kalıcı olarak yüklü gruplar arasındaki elektrostatik çekimlerdir. İyonik bağlar yönlendirilmez. Misal:
- X-CO2- ..... H3+N-Y
- ~ 20 kJ mol-1
Hidrojen bağları
[değiştir]Hidrojen bağları da elektrostatik çekimle kurulur, ancak kalıcı olarak yüklü gruplar arasında değil, bir grup içindeki atomların farklı elektronegatifliği 'nden kaynaklanan geçici olarak bir "dipol moment" ile yüklenen atomlar arasında oluşurlar. Hidrojen bağları iyonik bağlardan bile daha zayıftır ve genellikle düz bir çizgi boyunca oldukça yönlüdür. Biyokimyadaki en yaygın hidrojen bağları şunlardır:
- X-OH ..... O-Y
- X-OH ..... N-Y
- X-NH ..... O-Y
- X-NH ..... N-Y
Hidrojen bağları, 12-29 kJ mol-1 arasında bir enerjiye sahiptir.
Van der Waals etkileşimleri
[değiştir]Van der Waals etkileşimleri, elektron yoğunluğunun neden olduğu dipoller arasında kurulmuştur. İki atomun dış elektron kabukları neredeyse (ama tam olarak değil) temas ettiğinde oluşurlar. Bu zayıf etkileşimler için atomların uzaklığı çok önemlidir. Atomlar birbirinden çok uzaksa, etkileşimler kurulamayacak kadar zayıftır; atomlar birbirine çok yakınsa elektron kabukları birbirini iter. Van der Waals etkileşimleri oldukça belirsizdir; hemen hemen her iki atom arasında meydana gelebilirler. Enerjileri 4-8 kJ mol-1 arasındadır.
Hidrofobik kuvvetler
[değiştir]Hidrofobik kuvvetler gerçekte bağ değildir, bu nedenle bu listede dört öğe vardır, ancak yine de yalnızca üç bağ türü vardır. Su gibi polar bir çözünen madde için, polar olmayan bir molekülle olası bir hidrojen bağı kurmak enerjik olarak elverişsizdir. Bundan dolayı su, polar olmayan herhangi bir molekülün etrafında, o moleküle hiçbir hidrojen bağı göstermeyecek şekilde kendisini düzenler. Bu, "serbestçe" hareket eden suya kıyasla daha düzenli bir su ile sonuçlanır, bu da daha düşük bir entropi düzeyine yol açar. Bu durum enerji açısından elverişsizdir. Çözünen madde içinde birden fazla apolar molekül varsa, apolar moleküller tek bir yerde toplanarak su ile temas alanlarını minimize ederler. Ayrıca, proteinler gibi büyük moleküllerde, molekülün hidrofobik (apolar) kısımları içe doğru dönerken, polar kısımlar molekülün yüzeyine doğru dönme eğiliminde olur.
Kaynakça
[değiştir]Cooke, Rosa-lee. Properties of Water. Lecture 10. Mountain Empire Community College. n.d. Web. http://water.me.vccs.edu/courses/env211/lesson10_print.htm
Kimball, John W.. Hydrogen Bonds. Kimball’s Biology Pages. Feb. 12, 2011. Web. http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/H/Hbonds_water.gif
Lower, Stephen. States of matter: Water and hydrogen bonding. General Chemistry Virtual Textbooks. 2009. Aug. 26, 2010. Web. http://www.chem1.com/acad/webtext/states/water.html
n.p. Covalent vs. Non-Covalent Bonds. n.d. http://www.pearsonhighered.com/mathews/ch02/c02cv.htm
W. W. Norton & Company. Hydrogen Bonding in Water. Web. 2012. http://www.wwnorton.com/college/chemistry/gilbert2/tutorials/chapter_10/water_h_bond/
WyzAnt Tutoring. WyzAnt Tutoring. Bonds. 2012. Web. http://www.wyzant.com/Help/Science/Chemistry/Bonds/

![{\displaystyle {pH}={pK}_{a}+log{[baz] \over [asit]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0cf85f105b9e72afc5ee8c3ef0d15f7b18bd79d0)